Evaporation Lab
In this lab you explored evaporation and evaporative *cooling*. Hopefully you were all *somewhat* startled that the thermometer wrapped in we
t paper toweling dropped several degrees *below* the dry thermometer.

How can we explain this?
Thermal energy
Absolute temperature is proportional to the average thermal energy of moving atoms. $$\frac 32 k_bT = \frac 12 m\langle v^2 \rangle$$
- The angled brackets, $\langle ... \rangle$ mean "the average of...". In this case, the average (speed squared)..
- $k_B$ is the Boltzmann constant. $k_B \approx 1.38\times 10^{-23}$ Joules / ${}^o$K.
- Absolute temperature is measured in ${}^o$ K, or degrees Kelvin.
- But 0 K is a much lower temperature than 0 C or 0 F. (See the comparison below of the three different temperature systems.)
- As the absolute temperature approaches 0, the average (speed^2) of atoms approaches 0. The mass, $m$, of an atom does not change as temperature changes. So it's the speed, $v$ that approaches zero. That is, all thermal motion of atoms would theoretically stop if the temperature could drop to $T=0$ K.
Absolute temperature
This is a comparison of the three most commonly used scales for measuring temperature.
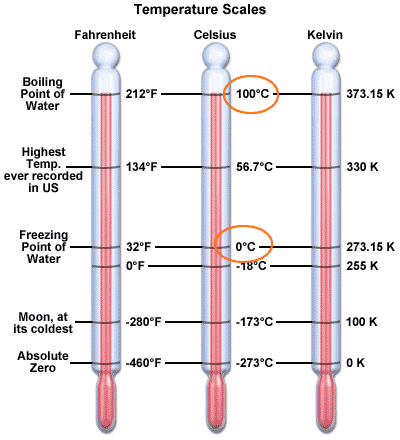
- How many kelvin degrees are there between 0 C and 100 C?
The difference between 100 C and 0 C is the difference between 373.15 K and 273.15 K. Both differ by 100 degrees. So, the size of a kelvin degree is the same size as a celsius degree. It's just that zero point is different.
How fast are atoms moving?
At room temperature, a typical speed is $v$ ~ 700 mi / h - close to the speed of sound.
 This
is no accident: Sound is a pressure disturbance travelling through air.
It seems plausible that any disturbance should move with the same underlying
speed as the atoms are moving.
This
is no accident: Sound is a pressure disturbance travelling through air.
It seems plausible that any disturbance should move with the same underlying
speed as the atoms are moving.
Evaporative cooling
The average human body temperature is 98.6 F = 37 C.
On a hot day, your body is sweating in hopes of cooling you down.

Matthew Kenwrick
Here's what's happening:
- Thermal energy says that temperature is proportional to the average speed^2 of molecules.
- But even if the temperature is steady, there are some molecules that are moving *faster* than average and others that are moving *slower* than average.
- Imagine that you have some liquid water on your body: Sweat! The fastest moving sweat (water) molecules are the most likely ones to escape from the liquid layer on your skin, and into the surrounding air.
- This leaves the slower moving ones behind.
- Since the fastest ones left, the average speed of the left-behind sweat molecules is lower, which is a fancy way of saying, the temperature of the sweat on your skin is lower.
This works best when there are very few water molecules in the air, that is when the air is dry, or the relative humidity is low.
In the vaporization lab, you calculated the latent heat of vaporization, $L_v=$ 540 calories per gram of water. This is the amount of energy carried off when 1 gram of water evaporates into water vapor.
But, as you calculated, when there are already many water molecules in the air, then as some of your sweat evaporates, other water molecules from the air are coming back to your sweat, reducing the effectiveness of evaporative cooling.
But when there are lots of molecules in the air, we have a dynamic equilibrium at 100% humidity: Just as many grams of water are evaporating as grams of water condensing.

This means that there is no cooling happening at 100% humidity.
If the air temperature (dry bulb) is 25 C and the humidity is 100%, what is the wet bulb temperature of a thermometer wrapped in a wet cloth?
It is harder to cool your body down by sweating as the humidity goes up.. A recent Penn State University study estimates that your body will overheat (can no longer keep its core temperature below 37 C) when the wet-bulb temperature reaches 31 C = 88 F.

Here's a simple way to blow cool air over yourself at home!

Water from the bucket wets a towel draped over the fan. As the fan pulls air through the wet towel, water from the towel evaporates, the towel cools, and cools the air passing through it. --Aaah!
Water vapor in a warming world
Hurricanes
The main prediction of global warming regarding hurricanes is more rain in the aftermath.
- Flash floods in Spain - "In the town of Chiva in the eastern Valencia region, practically a year’s worth of rain fell over eight hours".
- Hurricane Milton and Helene rainfall maps
Comparisons
Find what Goshen's annual rain fall is. How does this compare to the rainfalls from those hurricanes?
You found annual rainfall for Goshen around 39 inches / year.
Why?
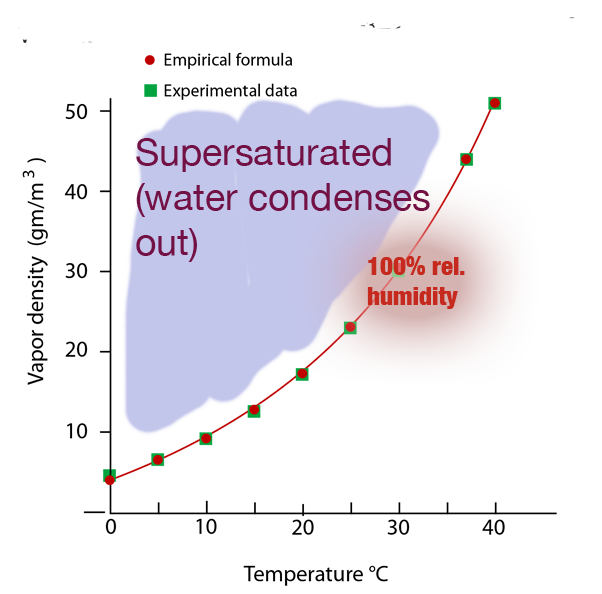
The "Saturation curve" for water vapor in Earth's atmosphere.
Higher temperatures and forest fires
Two effects of high temperatures work together to dry out vegetation and increase fire risk:
- High temperatures cause plants to pull up more water from the soil to sweat: "Evapotranspiration"--sometimes exhausting the supply of near-surface soil moisture.
- Once more the saturation curve:

...indicates that higher temperature air can absorb more moisture than cooler air before it reaches the limit of how much water it can carry off (reaches 100% humidity).
Test your understanding
-
The molecules in a container at which of these temperatures are moving the fastest?
- 0 ${}^o$F
- 0 ${}^o$C
- 0 ${}^o$K
- They are all moving with the same average speed.
show / hide answer
The molecules at 0 K are moving the slowest. Then, we know that 32 F = 0 C. So that means that 0 C is a higher temperature than 0 F. So 0 C is the highest temperature and so molecules at 0 C will have the fastest average temperature of these choices.
- Imagine that you have two molecules, one heavy and one light. If they both have exactly the same kinetic energy, which one is moving faster?
- The lighter one moves faster.
- The heavier one moves faster.
show / hide answer
Using $L$ and $H$ to mean light and heavy, if the two molecules have the same kinetic energy, that means: $$\frac 12 m_Lv_L^2=\frac 12 m_Hv_H^2.$$ $m_L$ is less than $m_H$. To make both sides of this equation equal, we therefore need $v_L$ to be greater than $v_H$, so the lighter molecule is moving faster
Using isotopes to measure pre-historic temperatures
There are three naturally occurring isotopes of oxygen which are not radioactive: ${}^{16}O$ (99.762% natural abundance), ${}^{17}O$, and ${}^{18}O$, which has a natural abundance of 0.20%=$r_0$.
All 3 are stable (none are radioactive). So, their relative proportions in the biosphere remain constant.
In the ocean, almost all of the water molecules, $H_2O$, contain the O-16 isotope, but a very small number of heavy water molecules have $H_2O$ molecules with O-18.
Evaporation and temperature

High
temps: at extremely high temperatures (boiling) all water molecules, both regular and heavy are equally likely leave the liquid and enter the air. So, the water in the atmosphere, and the water left behind would have:
$$r(\text{100 C})=\frac{\text{amount of heavy water}}{\text{amount of water}}= 0.20%=r_0$$
lower temperatures: But at lower temperatures, the fastest molecules are the most likely to leave the ocean, and that means the water molecules containing O-16 are the most likely to leave. So in warmer years:
- the atmosphere is enriched in oxygen-16 and snow falling in Greenland will be rich in oxygen-16, forming one ice layer per year.
- Oceans have the left-behind, heavier water, rich in O-18. That means marine life will get O-18 rich oxygen from seawater in their skeletons and shells. As that benthic (marine life) life dies, their skeletons / shells fall to the ocean bottom, each year piled on top of the previous year.
IPCC data for climate from years ago
...is based on measuring $\delta O-18$, changes to the relative amount of O-18 in ancient marine sediments, and in ancient layers of snow. The O-18 rich layers from benthic life (see graph below) indicate times when the temperature was very low.
'kya' means "kilo years ago". So, 100 kya means 100,000 years ago.